Bioequivalence: What It Means for Generic Drugs and Your Health
When you pick up a generic pill at the pharmacy, you might wonder: bioequivalence, the scientific standard that proves a generic drug performs the same as its brand-name version in the body. It's not just a label—it's the reason your insurance lets you pay $4 instead of $400 for the same condition. If two drugs are bioequivalent, they release the same amount of active ingredient into your bloodstream at the same speed. No guesswork. No hidden differences. Just the same effect, same safety profile, same outcome.
This matters because generic drugs, lower-cost versions of brand-name medications approved by regulators after proving they match the original make up 90% of prescriptions in the U.S. But not all generics are created equal—bioequivalence is the gatekeeper. Without it, a pill might not dissolve right, absorb too slowly, or cause side effects because your body reacts differently. That’s why regulators like the FDA require strict testing: blood levels must match within 80–125% of the brand-name drug. If it doesn’t pass, it doesn’t hit shelves.
And it’s not just about cost. generic substitution, when a pharmacist swaps a brand drug for a generic without re-prescribing is legal in most states—but only if bioequivalence is proven. Some drugs, like blood thinners or seizure meds, have tight windows for effectiveness. A tiny difference in absorption could mean a clot, a seizure, or worse. That’s why pharmacists check state rules and why patients should know: if your doctor says "dispense as written," it’s because they trust the exact formulation.
Behind every generic drug you take is a lab test measuring how fast and how much of the drug enters your blood—this is drug absorption, how quickly and completely a medicine enters your bloodstream after taking it. It’s why two pills that look identical can behave differently if their fillers or coatings change. Bioequivalence studies use healthy volunteers, track blood samples over hours, and compare curves. If the curves overlap closely enough, the generic gets approved.
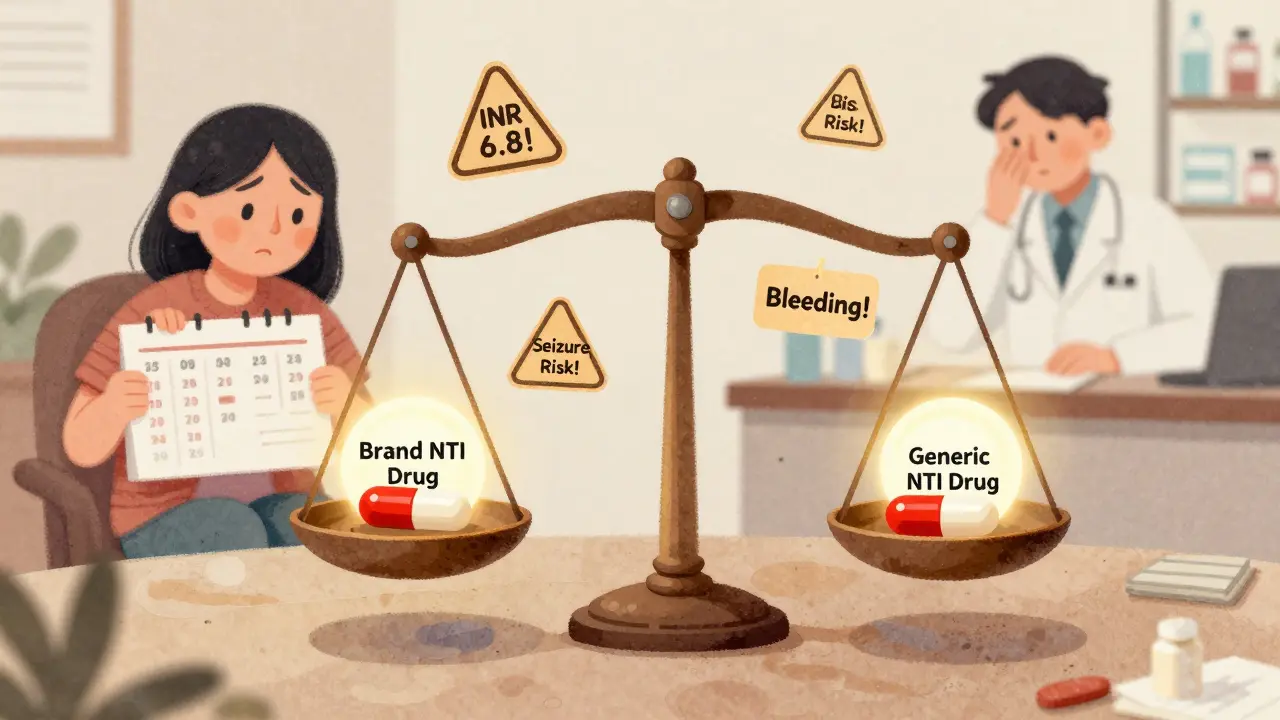
And here’s the thing: bioequivalence doesn’t mean the pills are identical. They can have different colors, shapes, or inactive ingredients. But the active part? That’s locked in. That’s why switching between generic brands is usually safe—if they’re both bioequivalent to the same brand. But if you’re on a narrow-therapeutic-index drug like warfarin or levothyroxine, sticking with the same generic brand matters more. Why? Because even small shifts in absorption can add up over time.
So when you see "generic" on your receipt, you’re not getting a cheaper knockoff—you’re getting a drug that had to prove it works just like the expensive one. And that’s not just marketing. It’s science. It’s regulation. It’s what keeps millions of people on affordable meds without sacrificing safety.
Below, you’ll find real-world examples of how bioequivalence plays out in daily care—from state laws forcing substitutions to how insurers pick which generics to cover, why some drugs get 180-day exclusivity, and what happens when a patient switches brands mid-treatment. This isn’t theory. It’s your prescription. And you deserve to know how it works.
ANDA Process: Legal Requirements for Generic Drug Approval in the U.S.
- Beata Staszkow
- |
- |
- 11
The ANDA process lets generic drug makers get FDA approval by proving bioequivalence to brand-name drugs, cutting costs and speeding access. Legal requirements include identical active ingredients, strict CMC standards, patent certifications, and GDUFA fees.
View morePopulation Pharmacokinetics: How Data Proves Drug Equivalence
- Keith Ashcroft
- |
- |
- 0
Population pharmacokinetics uses real-world patient data to prove drug equivalence, especially for vulnerable populations. Learn how it's replacing traditional bioequivalence studies and gaining global regulatory approval.
View moreCombination NTI Drugs and Generic Availability: Coverage and Gaps
- Keith Ashcroft
- |
- |
- 12
Combination NTI drugs offer powerful treatment for complex conditions, but generic versions are nearly nonexistent due to strict bioequivalence requirements and high safety risks. Patients face gaps in access and monitoring, with real-world consequences.
View moreHow the FDA Ensures Generic Drug Safety Through Manufacturing Oversight
- Keith Ashcroft
- |
- |
- 13
The FDA ensures generic drug safety through strict manufacturing standards, bioequivalence testing, and global inspections. Learn how generics are monitored from factory to pharmacy.
View more